A variety of external stress fields (mechanical, electrical…) or internal activity are ubiquitous in the practical situations under which lipid bilayers are assembled, transformed or utilized. Under these conditions the state of the membrane cannot be described by equilibrium statistical physics. Understanding far from equilibrium lipid and bilayer dynamics is one of the strongest challenges laying ahead for this field.
Biolubrication: the lubrication boundary of articular cartilage is a typical practical situation where these concepts come to play. The challenge here resides in the possibility of explaining and reproducing the conditions that lead to the robustness and low friction of this bio-lubricating contact. Supported stacks of lipid bilayers have been shown to provide a biomimetic system that displays some of the sought lubricating properties. In these systems, we will study the bilayer at different length-scales to understand the hydrodynamics of sheared confined layers and the bilayer friction with a solid polymer substrate.
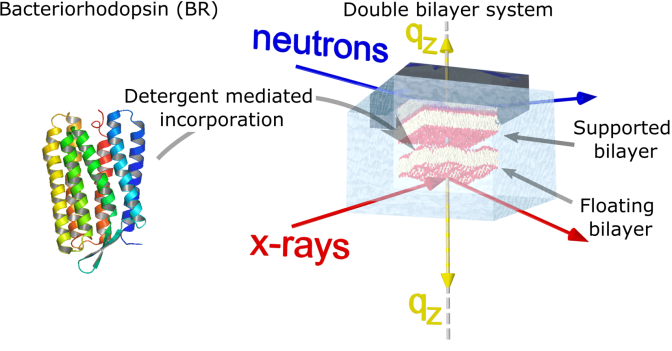
Active membrane: transmembrane proteins have a pivotal role in a large number of cellular processes and provide essential functions of the cell membranes such as inter- and intra-cellular signalling, transfer of substances inside and outside the cell, the transfer of ions through the membrane, energy transfer, cell homeostasis. By itself membranes exhibit thermal fluctuations, but membrane protein activity leads to out-of-equilibrium fluctuations, which break the fluctuation-dissipation theorem. Active fluctuations have been widely described theoretically, but less is known on the experimental point of view. First experiments on bacteriorhodopsin (BR), a light-activated proton pump reconstituted in Giant Unilamellar Vesicles, were performed by the group of Patrica Bassereau (Curie Institut), by using Micropipette aspiration and refined microscopy analysis. Despite these experiments show clear evidence for the magnification of shape fluctuations when proteins are activated, the experimental technique accesses only micrometer scale information and did not provide a measurement of the fluctuation spectrum. A complete understanding of the mechanism requires a fine characterization of the fluctuation spectrum at submicron length scales, which we seek to do by studying the fluctuations of active supported membranes through off-specular x-ray scattering experiments.
Within the PhD work of T. Mukhina (see here), we manage to insert bacteriorhodopsin into planar floating lipid bilayers in gel and fluid phases, by applying a detergent-mediated incorporation method (Mukhina et al). Neutron and X-ray reflectometry were used on both single and floating bilayers with the aim of determining the structure and composition of this membrane-protein system before and after protein reconstitution at sub-nanometer resolution. Lipid bilayer integrity and protein activity were preserved upon the reconstitution process. Reversible structural modifications of the membrane, induced by the bacteriorhodopsin functional activity triggered by visible light, were observed and characterized at the nanoscale.